Research Contents
We are conducting cutting-edge research with the responsibility to contribute to the science as well as society, and aims to foster a full-fledged physician and researcher who can significantly involve in the development of medicine and medical care in the upcoming days. Therefore, we conduct collaborative research with many research laboratories across the country and abroad, and perform basic and clinical investigations targeting a wide range of topics. Since there are many foreign graduate students and post-doctorals enrolled in our laboratory from different parts of the world (Thailand, Bangladesh, China), all the meetings are held in English that creates an international atmosphere.
1. Elucidation of the regulatory mechanism of renin-angiotensin-aldosterone system and its involvement in the pathogenesis.
2. Regulation of salt and body fluid in pathophysiological conditions
3. In vivo imaging in kidneys
4. Development of cancer therapy targeting the (pro)renin receptor.
5. Elucidation of the involvement of cell senescence in renal diseases
6. Elucidation of the involvement of sympathetic nerve activity in the development of hypertension in cardiovascular and renal diseases
7. Evaluation of the pharmacological properties of novel drugs.
1. Elucidation of the regulatory mechanism of renin-angiotensin-aldosterone system and its involvement in the pathogenesis
The key research goals are --Understanding the regulatory mechanisms of renin-angiotensin-aldosterone system in the kidney by elucidating the pathological changes and subsequently to develop a new treatment strategy.
-Identification of urinary bio-markers targeting the renin-angiotensin-aldosterone system for the development of new diagnostic methods for kidney related diseases.
-Conducting collaborative research by measuring the urinary bio-markers in the samples from other research groups around the world.
-Collaborating actively with other research groups and companies to promote our activities in Japan and abroad.
2. Rethinking of salt and body fluid balance concept
Do we increase water intake after we eat salty food? Can we calculate daily salt intake from 24 hours urinary Na+ excretion? We may miss something important.Osmolyte such as sodium ion (Na+) is an important factor in the maintenance of body fluid. Therefore, abnormal Na+ and water balance leads to dehydration, edema, and high blood pressure, which ultimately causes brain, cardiovascular, and kidney diseases. We are now challenging to answer the following questions from the perspective of a novel Na+ and water balance concept: Why consuming too much salt is not good for our health? How does our body regulate Na+ and water balance? Are they simple? In ultra-long-term salt and water balance study in healthy men, we found that "salty food decreases water intake and increases appetite," and that "urinary Na+ excretion is not always constant even when salt intake is constant.” (Kitada et al. JCI. 2017; https://pubmed.ncbi.nlm.nih.gov/28414295/) We couldn’t explain these findings by the current salt and water balance concept, therefore require a novel alternative concept. Our final goal is that we can make a healthy life in conjunction with salt, one of the best tasty seasonings.
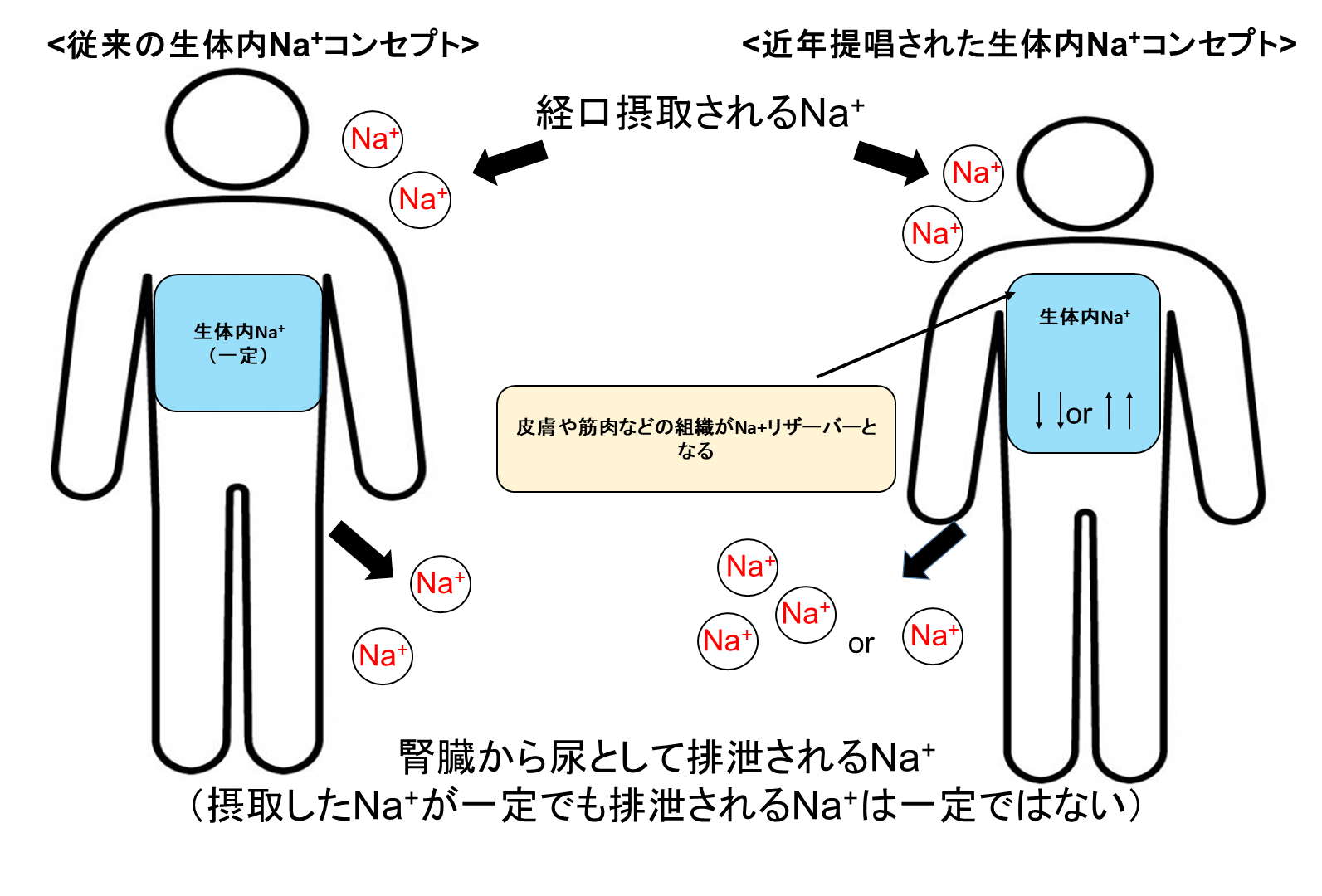 2-1. It is believed that 24 hours urinary Na+ excretion is almost equal to daily Na+ intake because the kidneys excrete excess salt into the urine.
However, the long-term salt and body fluid balance study (for more than 100 days) showed that even when salt intake is fixed, urinary Na+ excretion does not remain constant and Na+ accumulation occurs in tissues.
Our research questions: How do the tissues locally accumulate Na+?
Is there any good/bad effect on the tissues that accumulate/lose Na+?
2-1. It is believed that 24 hours urinary Na+ excretion is almost equal to daily Na+ intake because the kidneys excrete excess salt into the urine.
However, the long-term salt and body fluid balance study (for more than 100 days) showed that even when salt intake is fixed, urinary Na+ excretion does not remain constant and Na+ accumulation occurs in tissues.
Our research questions: How do the tissues locally accumulate Na+?
Is there any good/bad effect on the tissues that accumulate/lose Na+?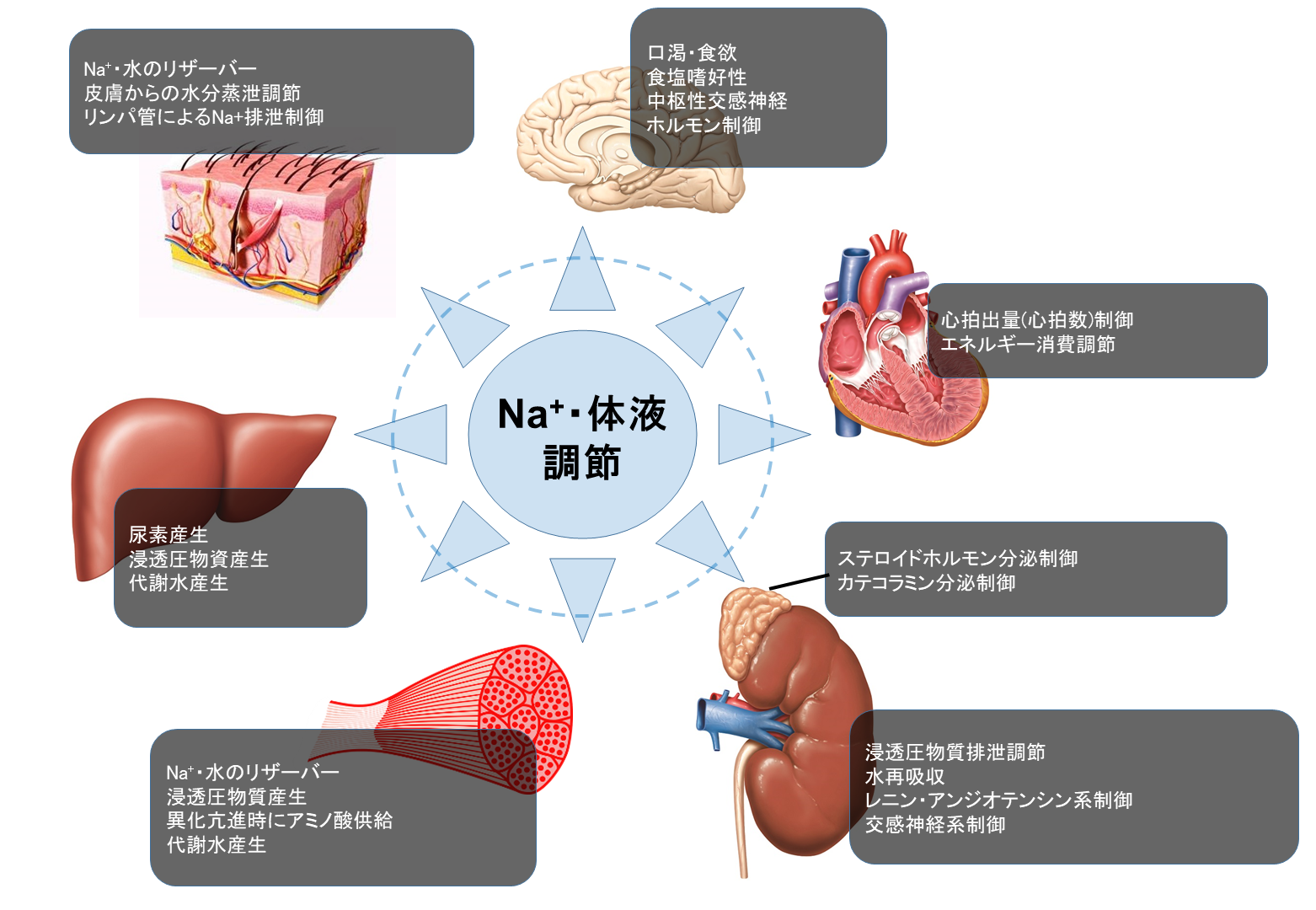 2-2. We have learned at school that the kidneys regulate Na+ and fluid balance in the body.
However, we found that the Na+ and water balance is maintained by various organs including kidney, skin, immune cells, liver, muscle, and heart.
We are now trying to understand the detailed mechanism of Na+ and water handling in numerous organs.
2-2. We have learned at school that the kidneys regulate Na+ and fluid balance in the body.
However, we found that the Na+ and water balance is maintained by various organs including kidney, skin, immune cells, liver, muscle, and heart.
We are now trying to understand the detailed mechanism of Na+ and water handling in numerous organs. 2-3. Joint Research with JAXA Focusing on Skin Function
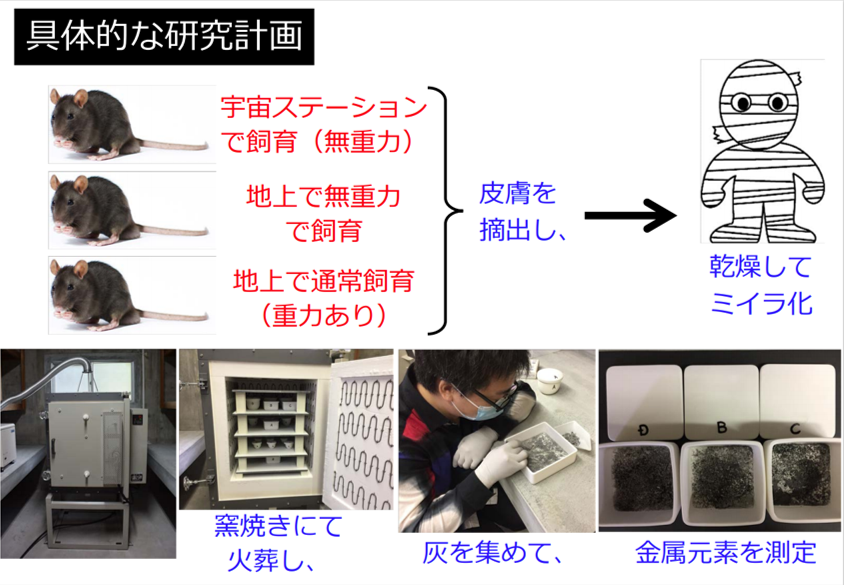
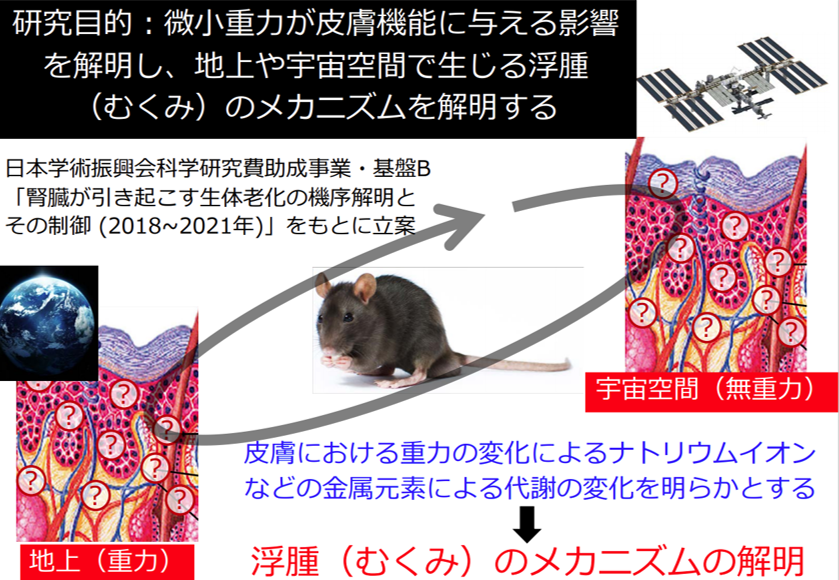
Kagawa University and the Japan Aerospace Exploration Agency (JAXA) have formally concluded a joint research agreement and have been conducting research aimed at "healthier life in space. Using mice raised in the Japanese Experiment Module "Kibo" on the International Space Station (ISS), we are verifying the changes in skin moisture, sodium ions and other metallic elements caused by microgravity.
Enlarge the slide

3. In vivo imaging in kidneys ? Did you see that! Yes, I saw it!
The idea is to look at the organs of living animals and discover function that is not known yet. Conventional physiological and pharmacological experiments have focused on assessing the functions upon administering drugs to animals or in isolated organs. Recently, it has been adopted to see the changes of drugs effects in genetically modified animals by analyzing and comparing the different parameters in blood and fixed tissue samples. Even though it is possible to assess the phenomenon occurring in the body by the conventional methods, still we have question like, "Can we see the physiological and pharmacological phenomena actually happening in the body in real time? The answer might be "We believe" and "data indicates" to the question. However, recent advances in microscopy have been allowing us to see the actual phenomenon happening in the tissues. This means that the conventional methods of "organ function analysis", "blood parameter analysis", and "tissue morphology analysis" can be performed simultaneously. "Do you really see it?" and "Yes, Sir!" it is now possible!We are working on in vivo imaging by multiphoton laser microscope in rats and mice. We have summarized our recent findings as follows:
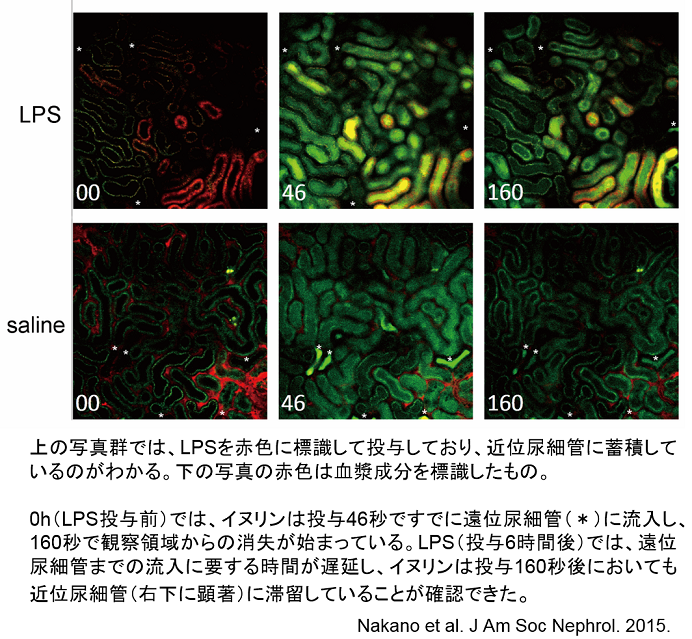
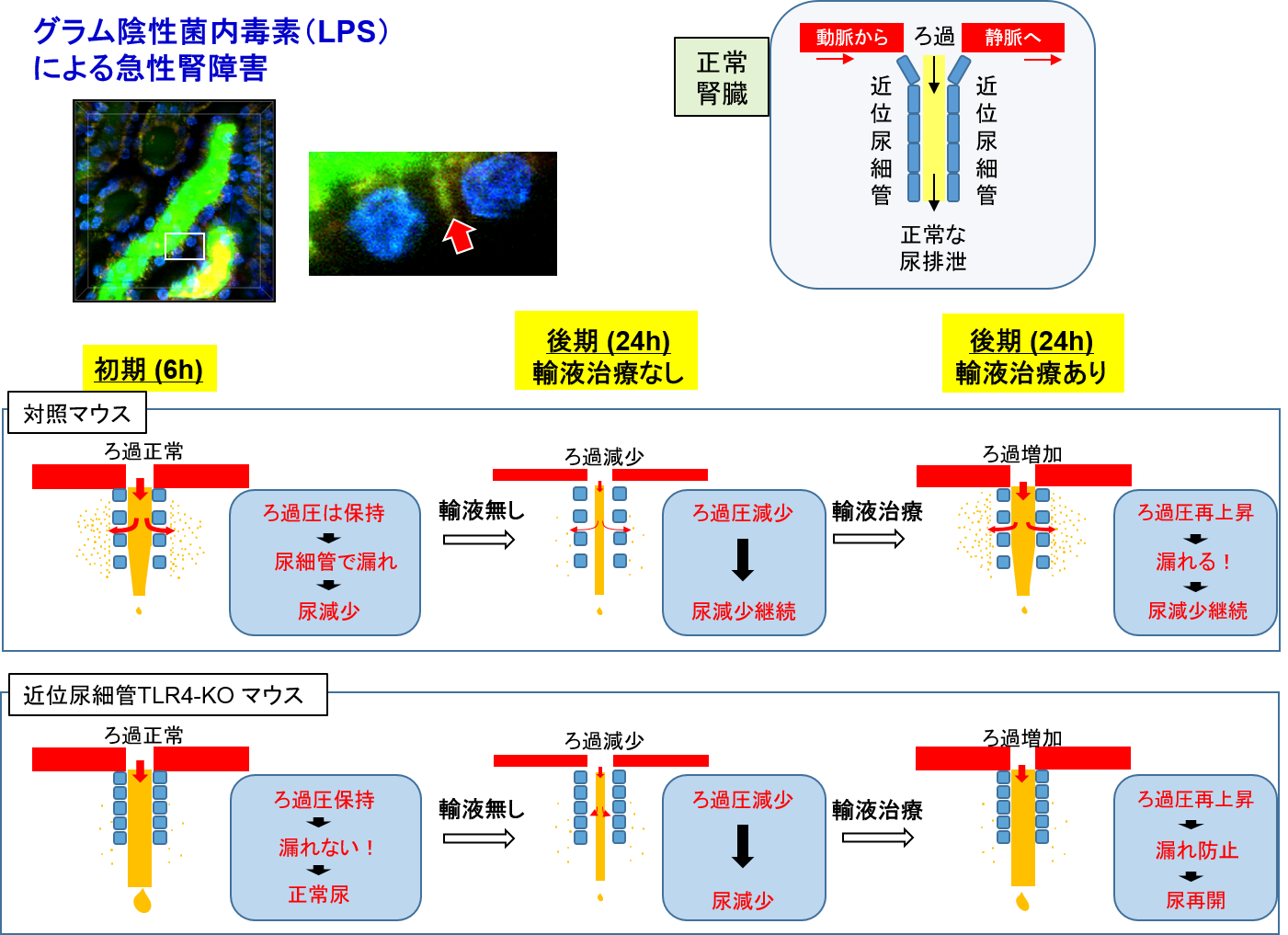
3-1. During sepsis, the kidneys rapidly lose their function and produce extremely less amount of urine (oliguria/anuria in acute kidney injury). In accordance with this established knowledge, we have demonstrated a decrease in urine flow rate in the first part of the long tubules (proximal tubular lumen) (video link below). This phenomenon was captured by live imaging and found that water (urine) leaked out due to a hole in the tubular lumen, which is like a hole in water-hose, even the glomerular filtration, which is a faucet for urine, is normal. This is considered to be a breakthrough as a new mechanism for the onset of acute kidney injury, which currently has no targeted treatment (Nakano et al. J Am Soc Nephrol. 2015 https://www.ncbi.nlm.nih.gov/pubmed/25855781). Furthermore, we have succeeded in clarifying several questions regarding the empirically used drug (natriuretic peptide, hANP) as follows. "How long after the onset of acute renal injury could the drug be effective, and from what stage it is difficult to treat” and “which cells are hard to treat" (Kitamura et al. Anesthesiology. 2018, https://www.ncbi.nlm.nih.gov/pubmed/29629958).
 3-2. Diabetic kidney disease is an important priority in the field of kidney disease research, and therefore, we must explore best therapeutics to combat it.
Recently, hypoglycemic drugs that act on the proximal tubular cells of the kidney (Sodium Glucose co-Transporter 2 inhibitors (SGLT2 inhibitors) have become available for clinical use.
Physiologically, blood sugar is filtered by the glomerulus, and almost all of this is taken up by the proximal tubules and returned to the blood.
SGLT2 inhibitors inhibit glucose uptake by the proximal tubule and promote glucose excretion in the urine, thereby reducing the amount of sugar in the body (i.e., lowering blood glucose levels).
Since the drug mainly work on kidney, the person who has extreme low level of the kidney function, hypoglycemic action cannot be expected.
So, use of SGLT2 inhibitor is refrained in these patients.
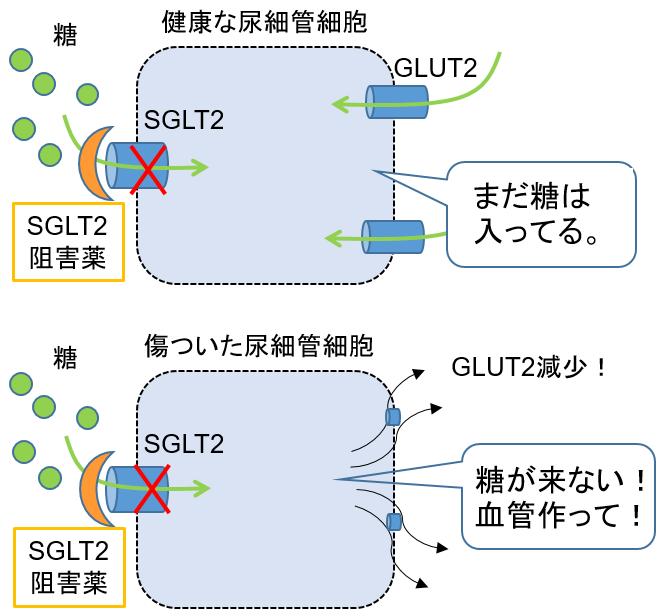
Since SGLT2 inhibitors inhibit sugar uptake into tubular cells, we hypothesized that this would cause starvation of cells for sugar and promote angiogenesis.
Our data revealed that SGLT2 inhibitors do not cause sugar starvation in normal tubular cells, but only in injured tubular cells to advance the process of angiogenesis.
By visualizing sugar uptake in the kidneys in alive mice, we demonstrated that SGLT2 inhibitors are effective in assisting the repair process of injured kidneys by protecting the capillary network
(Zhang et al. Kidney International 2018, https://www.ncbi.nlm.nih.gov/pubmed/30045814).
3-2. Diabetic kidney disease is an important priority in the field of kidney disease research, and therefore, we must explore best therapeutics to combat it.
Recently, hypoglycemic drugs that act on the proximal tubular cells of the kidney (Sodium Glucose co-Transporter 2 inhibitors (SGLT2 inhibitors) have become available for clinical use.
Physiologically, blood sugar is filtered by the glomerulus, and almost all of this is taken up by the proximal tubules and returned to the blood.
SGLT2 inhibitors inhibit glucose uptake by the proximal tubule and promote glucose excretion in the urine, thereby reducing the amount of sugar in the body (i.e., lowering blood glucose levels).
Since the drug mainly work on kidney, the person who has extreme low level of the kidney function, hypoglycemic action cannot be expected.
So, use of SGLT2 inhibitor is refrained in these patients.
Since SGLT2 inhibitors inhibit sugar uptake into tubular cells, we hypothesized that this would cause starvation of cells for sugar and promote angiogenesis.
Our data revealed that SGLT2 inhibitors do not cause sugar starvation in normal tubular cells, but only in injured tubular cells to advance the process of angiogenesis.
By visualizing sugar uptake in the kidneys in alive mice, we demonstrated that SGLT2 inhibitors are effective in assisting the repair process of injured kidneys by protecting the capillary network
(Zhang et al. Kidney International 2018, https://www.ncbi.nlm.nih.gov/pubmed/30045814).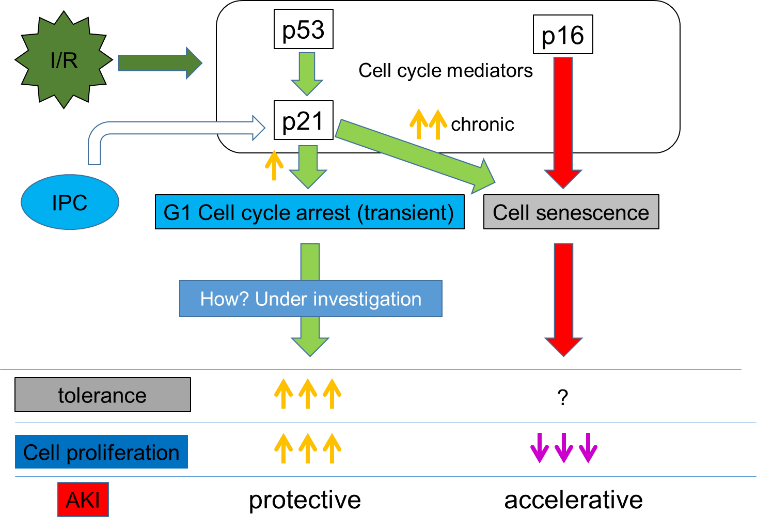 3-3. In acute kidney injury, death and regeneration of proximal tubular cells are commonly seen.
The surviving cells are thought to proliferate and regenerate the tubules.
Using mice that visualize the cell cycle (Tg(FucciG1)#596Bsi), we demonstrated that in acute kidney injury, transient cell cycle arrest is a protective mechanism against progression of injury and an essential phenomenon for the subsequent re-division and regeneration of cells.
(Nishioka et al. Kidney International 2014, https://www.ncbi.nlm.nih.gov/pubmed/24336034).
3-3. In acute kidney injury, death and regeneration of proximal tubular cells are commonly seen.
The surviving cells are thought to proliferate and regenerate the tubules.
Using mice that visualize the cell cycle (Tg(FucciG1)#596Bsi), we demonstrated that in acute kidney injury, transient cell cycle arrest is a protective mechanism against progression of injury and an essential phenomenon for the subsequent re-division and regeneration of cells.
(Nishioka et al. Kidney International 2014, https://www.ncbi.nlm.nih.gov/pubmed/24336034).3-4. The part of the body that filters blood to make urine (glomerulus) has a barrier mechanism that allows molecules necessary for the body to remain in the blood. Angiotensinogen (AGT), a major protein in the renin-angiotensin system that plays a major role in blood pressure regulation, is nearly the same size as albumin, yet AGT has a much smaller glomerular sieving than albumin (which is remain unfiltered). We have proposed that AGT in urine is a marker of renal impairment (Research Topic 1 described above), and this finding suggests that urinary AGT and albumin have different origin as the protein excreted in urine (Nakano et al. J Am Soc Nephrol. 2012, https://www.ncbi.nlm.nih.gov/pubmed/22997258).
3-5. Collaborative research
3-5-1. Collaboration with Bristol University in the UK
The glycoprotein layer referred as glycocalyx, which grows on the surface of glomerular capillaries, has been shown to be an important component in the underlying mechanism of for the disruption of the glomerular filtration barrier. Loss of this glycoprotein layer due to stress is considered to be one of the causes of protein leakage from the glomerulus. (Salmon et al. J Am Soc Nephrol. 2012, https://www.ncbi.nlm.nih.gov/pubmed/22797190).
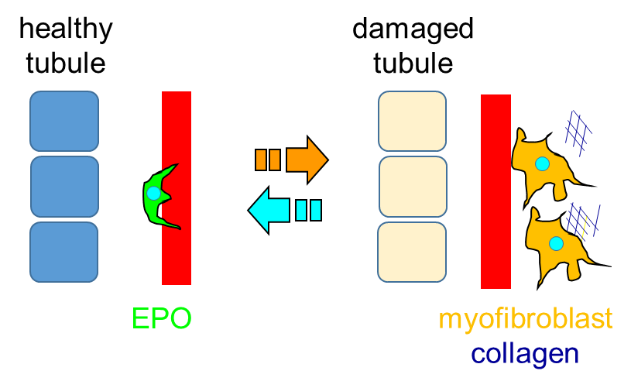 3-5-2. Collaborative research with Tohoku University
3-5-2. Collaborative research with Tohoku UniversityThe cells that produce erythropoietin, a hormone essential for red blood cell production, are located in the kidney. It has become apparent in the last decade that following renal injury these cells stop to produce erythropoietin (the cause of renal anemia), while producing collagen, which exacerbates renal fibrosis. Using mice with fluorescent tagging of the erythropoietin-producing cells, we have successfully visualized how these cells change their morphology and alter cellular function during renal injury progression (Souma et al. J Am Soc Nephrol. 2016, https://www.ncbi.nlm.nih.gov/pubmed/26054543).
3-5-3. Collaborative research with Niigata University
The cause of the nephrotoxicity associated with obesity, such as excessive fat intake, has not been elucidated in detail. There is a scavenger (megalin) in the proximal tubule to take up the proteins in the primary filtrate in order to reintroduce the proteins that have passed through the glomerular filtration barrier into the blood. It has demonstrated that this megaline-mediated lipid uptake leads to the accumulation of many wastes in the proximal tubule, causing the tubule to become dysfunctional and hypertrophic, and also triggering the compression of the interstitial region leading to the disruption of capillary flow (Kuwahara et al. J Am Soc Nephrol. 2016, https://www.ncbi.nlm.nih.gov/pubmed/26534923).
3-6. Movies
・・・Images of normal or septic mice injected intravenously with a fluorescent dye (green-yellow) that passes freely through the glomerulus. In normal mice, concentrated fluorescent dye can be seen in the distal tubules within 30-50 seconds, whereas in septic mice, concentrated urine is observed in the distal tubules more than 100 seconds after retention in the proximal tubules (noticeable at 30-60 seconds). Translated with www.DeepL.com/Translator (free version)
4. Development of cancer therapy targeting the (pro)renin receptor.
・ The (pro)renin receptor ((P)RR) was identified as a component of the renin-angiotensin system in 2002. Subsequently, it has also discovered that it is an essential component for Wnt signaling (Science 2010). Therefore, we have begun studying the probable link of (P)RR with that of cancer. We demonstrated that overexpression of (P)RR activates the Wnt/?-catenin signaling pathway in pancreatic cancer, brain cancer and colorectal cancer and is involved in the pathogenesis of the tumor. Furthermore, we have found that soluble (P)RR levels in the blood are elevated in the early stage of some cancer patients (applied for patent).・ In addition, with the aim of developing an antibody therapy against (pro)renin receptor, we generated a monoclonal antibody that blocks Wnt/β-catenin signaling and inhibits the growth of cancer cells. Our experimental data suggest that the generated monoclonal antibody could inhibit the growth of cancer cells (applied for the patent) (Rahman et al. MCT 2020). We are currently developing various (pro)renin receptor inhibitors to test their efficacy against diseases in which activation of the Wnt/β-catenin signaling pathway is central to the pathogenesis.
・ In addition, we have recently shown that abnormalities in (pro)renin receptor expression are involved in genomic instability, and we believe that this could be a new diagnostic and therapeutic target for various cancers and congenital diseases. Regarding this, we are now conducting translational research for clinical application with the support of AMED.
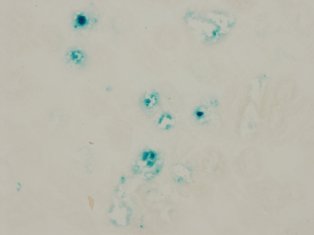
5. Elucidation of the involvement of cellular senescence in renal disease
・Elucidation of the role of cellular senescence in kidney diseaseIn diabetes and hypertension, as the disease progresses, blue stained tubules appear in the kidneys as shown in the picture. These cells are considered as "senescent cells" and it is known that such cells appear not only in aging but also in the diseases mentioned above. So what are the function of these senescent cells? Are these blue stained cells behave like normal cell? Or does the presence of these cells have adverse effects in the kidney? The answer is, “we are not sure yet”. Our goal is to figure out whether these "aging cells" are playing a good role or a bad role or not doing anything at all.
As part of this, we reported that a variety of stresses cause aging of proximal tubules of the kidney, resulting in attenuated tubular self-repair capacity, induction of apoptosis, and promotion of fibrosis (Endocrinology, 2011, Am J Hypertens 2012, J Pharmacol Sci 2012, Clin Exp Pharmacol Physiol. 2012, J Diabetes Complications 2014). These changes caused by cellular senescence are thought to be closely related to the decline in renal function in hypertension and diabetes. In addition, it is known that renal function deteriorates in humans with the advancing of aging. The proximal tubular cells are not exception. One of the goals of this project is to clarify whether there is a link between ‘cellular senescence’ and ‘renal function decline due to aging’ and subsequent underlying molecular mechanisms. This is because tubular cells fall into a non-dividing state, that is, “cell senescence”, where waste products that should be diluted at the time of mitosis accumulate due to aging, and the lysis enzyme activity is increased for degrading it.
・Establishment of a new method to detect senescent cells
Recent studies showed that senescent cells are involved in the development of cardiovascular and renal diseases. However, the role and function of senescent cells in the pathogenesis of the diseases is not fully understood since there are few methods available to detect senescent cells (ex: senescence-associated β-galactosidase staining). Especially, the detection of senescent cells in living cells or animals is missing to understand the function and role of senescent cells. To overcome this limitation, we are challenging to develop a new method to identify senescent cells by applying "two-dimensional Fourier spectroscopy" and "optical tweezers technology" in collaboration with the Faculty of Engineering, Kagawa University.
As shown in the figure below, two-dimensional Fourier spectroscopy can analyze the spectral characteristics of individual living cells. The goal of this project is to discover the spectroscopic properties specific to senescent cells and identify living senescent cells. After identifying senescent cells, we will extract the senescent cells using "optical tweezers technology" that allows cells to move in a live state. Once we will get the senescent cells alive, it will be a great tool for us to analyze the functions and roles of senescent cells.